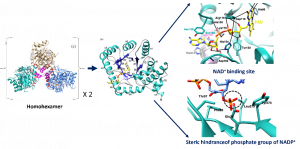
Methylenetetrahydrofolate reductase (MTHFR) is a key metabolic enzyme in colonization and virulence of Neisseria meningitidis, a causative agent of meningococcal diseases. In this study, we have reported the biochemical and structural properties of MTHFR from a virulent strain of N. meningitidis serogroup B (NmMTHFR). The three-dimensional structure revealed a unique homohexamer, which differed from other orthologs. The study of kinetic properties also revealed the difference in substrate preference between NADH for NmMTHFR and NADPH for human MTHFR. This could be explained by the different orientation of helix α7A, the extended sidechain of Met221 on helix α7A of NmMTHFR plays a role in stabilizing the folded structure of NADH in the hydrophobic box. While this restricts the phosphate group of NADPH that causes steric clashes with Glu26. This valuable finding can be applied to design antibacterial molecules without off-target that bind specifically to NmMTHFR, but not human MTHFR.
Reference
Pantong W, Pederick JL, Maenpuen S, Tinikul R, Jayapalan JJ, Jovcevski B, Wegener KL, Bruning JB, Salaemae W. Biochemical and structural characterization of meningococcal methylenetetrahydrofolate reductase. Protein Sci. 2023 Jun;32(6):e4654.
DOI: 10.1002/pro.4654.
| Relevant SDGs | |
|---|---|
| BC investigator | |
 Asst. Prof. Ruchanok Tinikul Asst. Prof. Ruchanok Tinikul |
|