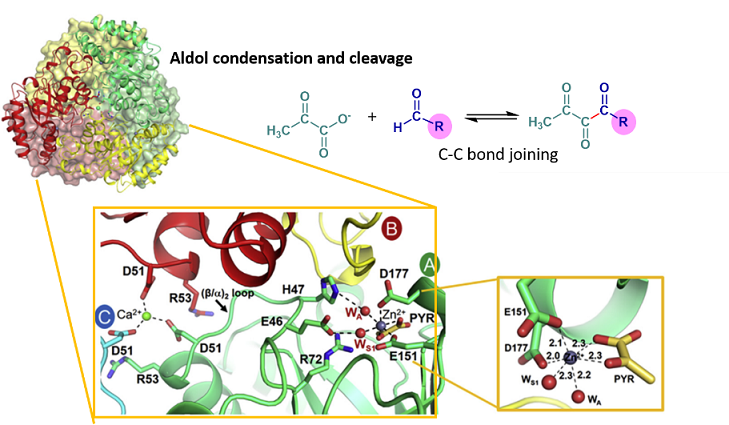
Aldolase catalyzes reversible C-C bond condensation and joining. Its reaction is useful in biochemical synthesis, participating in extending the carbon chain length of a target molecule. In this paper, we characterized a novel Class II metal aldolase, a member of the aromatic degradation pathway of Acinetobacter baumannii. The enzyme possesses stereospecificity, broad aldehyde utilization, high thermostability, and solvent tolerance. The X-ray structure complex with metal cofactors revealed the octahedral geometry of amino acids in active sites (Glu151 and Asp177), pyruvate, and water molecules, as well as the use of Zn2+ as a cofactor for the first time. The data also revealed a structural determinant of Arg72 that governs the stereoselectivity/stereospecificity of the enzyme.
This stereospecific aldolase with high thermal and solvent tolerances would be beneficial for biocatalytic applications
Reference
Watthaisong P, Binlaeh A, Jaruwat A, Lawan N, Tantipisit J, Jaroensuk J, Chuaboon L, Phonbuppha J, Tinikul R, Chaiyen P, Chitnumsub P, Maenpuen S. Catalytic and structural insights into a stereospecific and thermostable Class II aldolase HpaI from Acinetobacter baumannii. J. Biol. Chem. 2021, 297, 101280.
| Relevant SDGs | |
|---|---|
 |
|
| BC investigator | |
 Asst. Prof. Ruchanok Tinikul Asst. Prof. Ruchanok Tinikul |
