Cholangiocarcinoma Research Group
| Cancer is a disease in which cells divide abnormally, producing an excess mass of cells which can spread to distant organs via a process termed ‘metastasis’. Not all the cell mass, or the ‘tumor’, are capable of spreading. The ones that cannot spread are termed ‘benign tumor’, whereas those that can spreadare termed ‘cancer’. Metastasis is the major cause of death among cancer patients. Without metastasis, tumors can be easily eliminated by surgical resection or radiation therapy, rendering a high rate of the patient’s survival. However, if metastasis has taken place, the prognosis is poor due to growth of cancers in multiple organs, impeding the normal function of those organs that are vital to the survival of the patients. Currently there is no effective therapy for metastatic cancers. Identification of molecules and signaling pathways that play important roles in the metastatic process will be imperative to the suppression or reduction of metastasis, hence improving the chance to survive. Since cancer is a disease with multiple gene mutations, targeting just one molecule might not be sufficient and multiple targeting might be the key to successful suppression of the disease. |
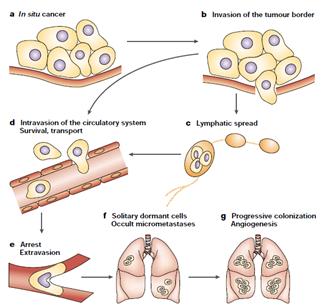 The initiation and progression of tumors depend on the acquisition of specific functions by cancer cells at both the primary and metastaticsites. Functions associated with tumor initiation are provided by oncogenic mutations and inactivation of tumor-suppressor genes.Functions associated with the initiation of metastasis include functions to which tumor cells resort for local invasion and for circumventinghypoxia and other limitations facing a growing tumor. Most functions for the initiation of both the tumor and metastasis remain essentialfor cancer cells to continue their metastatic development. Functions for metastasis progression provide a local advantage in aprimary tumor and a distinct and sometimes organ-specific function during metastasis. Cancer cells that are endowed with these threesets of functions still depend on functions associated with metastasis virulence; these functions confer a selective advantage solely duringthe adaptation and takeover of a specific organ microenvironment. Genes associated with each of these functions have been identified in recent years. (Steeg P, 2003) The initiation and progression of tumors depend on the acquisition of specific functions by cancer cells at both the primary and metastaticsites. Functions associated with tumor initiation are provided by oncogenic mutations and inactivation of tumor-suppressor genes.Functions associated with the initiation of metastasis include functions to which tumor cells resort for local invasion and for circumventinghypoxia and other limitations facing a growing tumor. Most functions for the initiation of both the tumor and metastasis remain essentialfor cancer cells to continue their metastatic development. Functions for metastasis progression provide a local advantage in aprimary tumor and a distinct and sometimes organ-specific function during metastasis. Cancer cells that are endowed with these threesets of functions still depend on functions associated with metastasis virulence; these functions confer a selective advantage solely duringthe adaptation and takeover of a specific organ microenvironment. Genes associated with each of these functions have been identified in recent years. (Steeg P, 2003) |
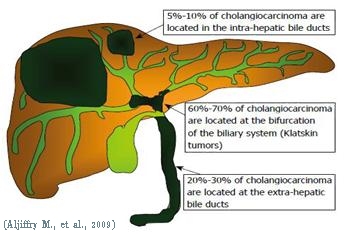
| Our group is particularly interested in defining how cancer cells metastasize. We want to decipher which molecules and signaling pathways are involved, and how these molecules orchestratein the metastatic process. The model that we used in our study is Cholangiocarcinoma (CCA) which is a cancer of the bile duct. Although considered a rare disease, the world-wide incidence rate of CCA has been increasing recently, and the incidence rate of CCA in northeastern Thailand is the highest in the world (average CCA incidence of 118.8 per 100,000 persons (Sripa B, 2007)).Importantly, it is a lethal disease due to high rates of local invasion and frequent regional lymph node metastasis. In addition, the survival rate of CCA patient is very poor, exhibiting a three-year survival of 35-50% in patients with histologically negative margins at the time of surgery. Since CCA in Thailand is associated with liver fluke (Opisthorchisviverrini) infection and chronic inflammation, it is believed that the fluke’s proteins as well as cytokines secreted by immune cells play a role in the metastatic development of CCA. These molecules can bind to receptors on the cell membrane, activating cascades of signaling pathways which culminate in the metastatic development. |
 Preparation of a meal of Koi-Pla using uncooked Cyprinoid fishes which is a common source of infection with O. viverrini. (Sripa B et al., 2007). Preparation of a meal of Koi-Pla using uncooked Cyprinoid fishes which is a common source of infection with O. viverrini. (Sripa B et al., 2007). |
 |
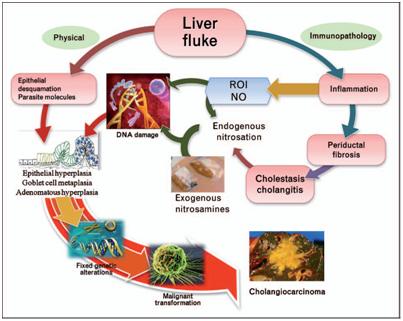 The liver fluke induces damage to the bile duct tissue at least by two pathways: mechanical (by parasite sucker/excretory–secretory products) andimmunopathology to parasite antigens. Inflammation-induced oxidative DNA damage occurs concurrent with biliary epithelial proliferation driven byparasite molecules. Damaged DNA/genes after successive replication become fixed and lead to malignant transformation. NO, nitric oxide; ROI,reactive oxygen intermediates. (Sripa B &Pairojkul C, 2008) The liver fluke induces damage to the bile duct tissue at least by two pathways: mechanical (by parasite sucker/excretory–secretory products) andimmunopathology to parasite antigens. Inflammation-induced oxidative DNA damage occurs concurrent with biliary epithelial proliferation driven byparasite molecules. Damaged DNA/genes after successive replication become fixed and lead to malignant transformation. NO, nitric oxide; ROI,reactive oxygen intermediates. (Sripa B &Pairojkul C, 2008) |
| Assoc. Prof. RutaiwanTohtong The research in my lab focuses on a number of molecules involved in distinct processes during the metastatic development of CCA. |
| Neutrophil gelatinase-associated lipocalin (NGAL)
Neutrophil gelatinase-associated lipocalin (NGAL), a 25 kDa glycoprotein which belongs to the lipocalin superfamily, is characterized by their low molecular weight and their ability to bind to and transport a variety of hydrophobic ligands such as fatty acids, retinoids and pheromones.Although originally identified in neutrophil as a covalent complex with gelatinase, NGAL was found to be expressed in numerous cell types including epithelial cells undergoing inflammatory reactions. The role of NGAL in various neoplastic human tissues has been controversial. Here, we showed that NGAL is required for the invasiveness of CCA cells by downregulating NGAL expression with siRNA and assessing the phenotypes of the silenced cells in vitro. Our data suggest that NGAL promotes the invasiveness of the cholangiocarcinoma cells by forming complex with MMP-9, stabilizing its activity and rendering the cancer cells to be more invasive. In addition, we performed immunohistochemical analysis of tumor specimens from CCA patients and related the in vivo expression pattern of NGAL to its role in regulating the invasiveness of the cancer cells in vitro. |
|
|
|
|
| CD44
CD44 is a membrane receptor which plays a dual role as an adhesion molecule that mediates cell-cell and cell-matrix adhesion, and as a receptor which transmits signals to the nucleus to regulatethe metastatic properties of the cancer cells.To metastasize, the cancer cells must detach from the primary tumor mass and form new interactions with the new environment, some of the interactions may be mediated by CD44. Contradictory roles of CD44 have been reported, where CD44 serves as a promoter in some cancer types, and as a suppressor in others. In CCA, we found that CD44 is a promoter of metastasis because when we knocked down CD44 using siRNA, the metastatic propertiesof the silenced cells were impaired. This suggested that CD44 plays an important role in metastasis andthat itmay serve as a molecular target for CCA treatment. We are currently exploring the other important metastatic properties regulated by CD44 and the underlying mechanisms that regulate these properties. |
|
|
|
|
|
|
| Gadd45b (Growth Arrest and DNA damage-inducible)
Gadd45b is a molecular signal that plays a role in the survival and death decision. Gadd45b has been shown to promote survival in some cancer types. One of the most serious problems of CCA is resistance to chemotherapeutic drugs. We hypothesized that the enhanced drug resistance is, at least in part, due to Gadd45b upregulation, hence, increasing survival signals. Indeed, our CCA cell lines expressed high level of Gadd45b, correlating with resistance to 5-FU and Cisplatin. Moreover, silencing Gadd45b expression using siRNA reduced resistance to the chemotherapeutic drugs due to increased apoptotic cell death, demonstrating that Gadd45b can perhaps be used as a molecular target to increase sensitivity of CCA to chemotherapeutic drugs. We are currently investigating the effect of Gadd45b silencing in different cell lines with different levels of resistance, as well as the effect on cell cycle, and the signaling pathways that are involved in this process. |
|
|
|
|

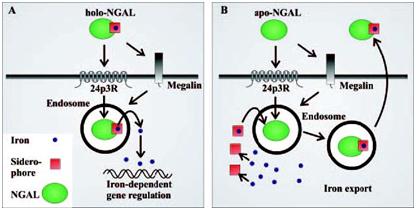 Schematic model of NGAL-mediated iron traffic. (A) Siderophore:iron-associated NGAL (holo-NGAL) delivers iron into the cell. After receptor-mediated uptake, NGAL traffics in acidic endosomes, which promote the release and cytoplasmic accumulation of iron, resulting in regulation of iron-dependent genes (9). (B) Siderophore:iron-free NGAL (apo-NGAL) captures intracellular iron and transports it to the extracellular space. Endosomal NGAL captures iron via a hypothetical intracellular siderophore, which is followed by recycling to the extracellular space (Schmidt-Ottet al., 2007).
Schematic model of NGAL-mediated iron traffic. (A) Siderophore:iron-associated NGAL (holo-NGAL) delivers iron into the cell. After receptor-mediated uptake, NGAL traffics in acidic endosomes, which promote the release and cytoplasmic accumulation of iron, resulting in regulation of iron-dependent genes (9). (B) Siderophore:iron-free NGAL (apo-NGAL) captures intracellular iron and transports it to the extracellular space. Endosomal NGAL captures iron via a hypothetical intracellular siderophore, which is followed by recycling to the extracellular space (Schmidt-Ottet al., 2007).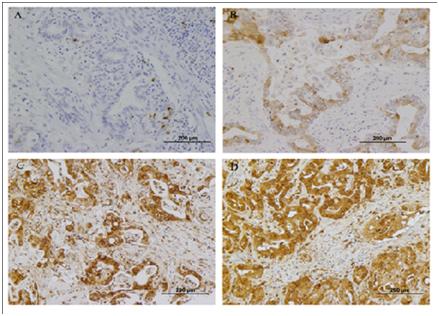 Expression analysis of NGAL by immunohistochemistry. NGAL was mainly localized within the cytoplasm of cholangiocarcinoma cells. A: Negative NGAL-staining in non-cancerous tissue (200?). B: Weak (1+) NGAL-staining in tumor cells (200?). C: strong NGAL staining (2+) in tumor cells (200?). D: strong NGAL staining (3+) in tumor cells (200?). (Nuntagowatet al., 2010).
Expression analysis of NGAL by immunohistochemistry. NGAL was mainly localized within the cytoplasm of cholangiocarcinoma cells. A: Negative NGAL-staining in non-cancerous tissue (200?). B: Weak (1+) NGAL-staining in tumor cells (200?). C: strong NGAL staining (2+) in tumor cells (200?). D: strong NGAL staining (3+) in tumor cells (200?). (Nuntagowatet al., 2010).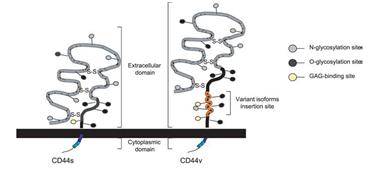 Structure of the CD44 molecules. The CD44 molecule is composed of an extracellular link domain, a stalk like region in the extracellulardomain close to the transmembrane region, where the variant exon products are inserted, the transmembrane region and the cytoplasmic tail.The link module contains the binding site for HA. The cytoplasmic tail contains binding motifs for the cytoskeletal linkerproteins ankyrin and ERM proteins as well as binding sites for the Src phosphokinases lck, lyn, and fyn that overlap the ankyrin- and ERM-bindingdomain (Marhaba, R. & Zoller, M., 2004)
Structure of the CD44 molecules. The CD44 molecule is composed of an extracellular link domain, a stalk like region in the extracellulardomain close to the transmembrane region, where the variant exon products are inserted, the transmembrane region and the cytoplasmic tail.The link module contains the binding site for HA. The cytoplasmic tail contains binding motifs for the cytoskeletal linkerproteins ankyrin and ERM proteins as well as binding sites for the Src phosphokinases lck, lyn, and fyn that overlap the ankyrin- and ERM-bindingdomain (Marhaba, R. & Zoller, M., 2004)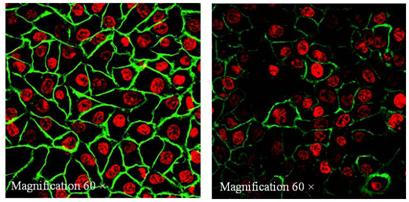 Immunocytochemical staining of CD44 using CD44-FITC monoclonal antibody which detected all forms of CD44 proteins revealed a strong and uniform staining in KKU-100 but a weak and punctated staining in HuCCA-1. Magnification 60X. Green color: FITC anti-human CD44, Red color: Topro3 (stained nucleus).
Immunocytochemical staining of CD44 using CD44-FITC monoclonal antibody which detected all forms of CD44 proteins revealed a strong and uniform staining in KKU-100 but a weak and punctated staining in HuCCA-1. Magnification 60X. Green color: FITC anti-human CD44, Red color: Topro3 (stained nucleus). In vitro invasion assay
In vitro invasion assay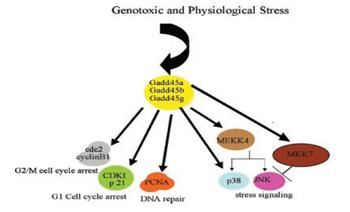 Summary of physical interactions of Gadd45 with other cellular proteins (Hoffman B & Liebermann DA, 2008).
Summary of physical interactions of Gadd45 with other cellular proteins (Hoffman B & Liebermann DA, 2008).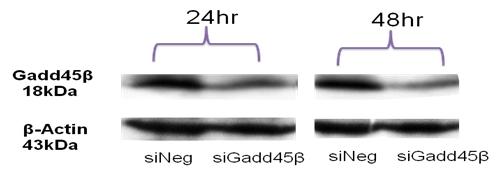 Silencing of Gadd45b gene expression using siRNA. At 24 h and 48 h post-transfection, Gadd45 b mRNA was significantly reduced as assessed by Western Blot. β -Actin was used as an internal loading control.
Silencing of Gadd45b gene expression using siRNA. At 24 h and 48 h post-transfection, Gadd45 b mRNA was significantly reduced as assessed by Western Blot. β -Actin was used as an internal loading control.