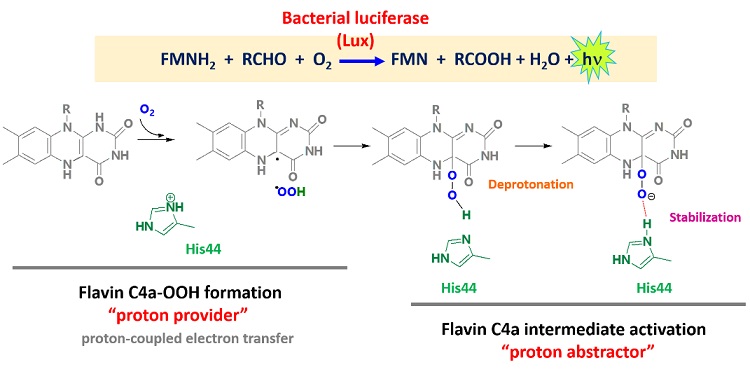
Bacterial luciferase (Lux) catalyzes light-emitting reactions, and the enzyme has long been used as a sensitive detection tool in biomedical, food, and environmental applications. In this report, our group has employed computer-based calculation, quantum mechanics (QM) and combined QM and molecular mechanics (QM/MM), to elucidate the molecular mechanism of FMNH-C4a−OOH formation and the role of His44 and its protonation state interchange in Lux catalysis. The results suggested the pathway of (1) H+ transfer coupled with electron transfer to O2 to generate +OOH, (2) the +OOH attacking at C4a of FMNH− to generate FMNH-C4a−OOH and (3) then His44 participating in accepting H+ from FMNH-C4a−OOH to form FMNH-C4a−OO− as a stable product stabilized by His44. As the light reaction crucially requires the deprotonated form of the FMNH-C4aOO− intermediate. This knowledge of the determinant factor to generate stable FMNH-C4a−OO− may be helpful for light brightness improvement, that is valuable for Lux application.
Reference
Lawan L, Tinikul R, Surawatanawong P, Mulholland AJ, Chaiyen P. QM/MM molecular modeling reveals mechanism insights into flavin peroxide formation in bacterial luciferase. J. Chem. Inf. Model. 2022, 62, 399−411.
| Relevant SDGs | |
|---|---|
 |
|
| BC investigator | |
 Asst. Prof. Ruchanok Tinikul Asst. Prof. Ruchanok Tinikul |
