Characterization of intra- and inter-species hybrid tetramers of pyruvate carboxylase: Biotin and the BCCP domain play a crucial role in determination of the kinetics and thermodynamics of catalysis
Khanti Rattanapornsompong, Sarawut Jitrapakdee1 and Paul V. Attwood2
1Department of Biochemistry, Faculty of Science, Mahidol University, Thailand
2 School of Molecular Sciences, The University of Western Australia, Perth, WA, Australia.
Source: Arch. Biochem. Biophys 695:108630
Pyruvate carboxylase (PC) (EC 6.4.1.1) is biotin-dependent carboxylase which catalyses carboxylation of pyruvate to form oxaloacetate using HCO3- and involving concomitant cleavage of MgATP. In the initial investigation of the relationship between the tetrameric structure and enzymic activity of Rhizobium etli PC, it has been demonstrated that inter-subunit catalysis occurs within pairs of subunits. Within each pair of subunits in the tetrameric structure of PC, the main pathway of catalysis in the presence of acetyl CoA involves biotin carboxylation in the biotin carboxylation (BC) domain of its own subunit. The carboxybiotin moiety is then translocated by the biotin carboxyl carrier protein (BCCP) domain to the carboxyltransferase (CT) domain of its partner subunit, where pyruvate carboxylation occurs [6,7]. Recently however, it has been reported that other types of inter-subunit catalysis are possible. Recently however, it has been reported that other types of inter-subunit catalysis are possible. For example, the biotin of one subunit may be both carboxylated and transfer its carboxyl group to pyruvate in its partner subunit’s BC and CT domains.
The aims of the current work were to use hybrid tetramers to further understand how PC functions as a tetramer and how the allosteric activator, acetyl CoA affects both the structure and activity of the PC tetramer. In addition, we provide further evidence that formation of hybrid tetramers between subunits of PCs from very evolutionarily diverse organisms is possible, resulting in hybrid tetramers that have some attributes of both PCs.
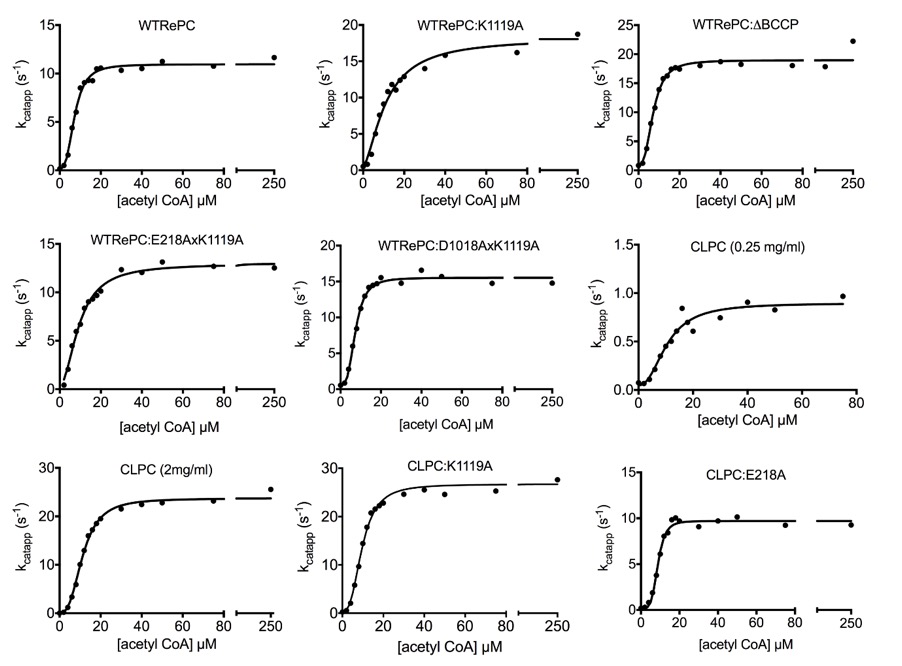
Figure 1 Shows plots of kcatapp versus concentration of acetyl CoA for WTRePC and 1:7 mixtures of the enzyme with K1119A, ΔBCCP, E218A.K1119A and D1018A.K1119A.
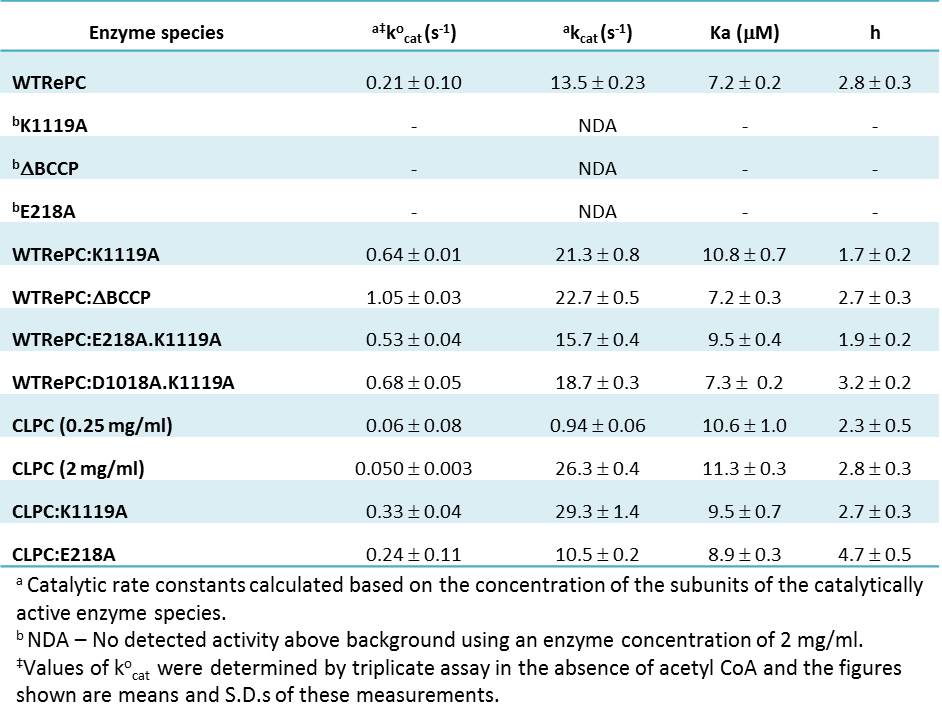
Table 1 shows the kinetic parameters obtained from fits of the data in Fig. 1 to equation (1). The mutant RePCs, K1119A and E218A, in this study had no detectable PC activity. The kocat and kcat values for all enzyme mixtures containing single or double mutants lacking biotinylation were significantly higher (kocat: 2.5-5-fold; kcat: 1.2-1.7-fold) than those of WTRePC alone (t-test; p<0.05). Ka values of the enzyme mixtures WTRePC:ΔBCCP and WTRePC:D1018A.K1119A were not significantly different from WTRePC alone. However, those of WTRePC:K1119A and WTRePC:E218A.K1119A were significantly greater than that for WTRePC alone. The values of the Hill coefficients (h) for the mixtures were similar to that of WTRePC alone, except those for WTRePC:K1119A and WTRePC:E218A.K1119A which were 39% and 22% lower respectively, than that of WTRePC alone.
Table 1. Kinetic parameters of pyruvate carboxylation at varied concentrations of acetyl CoA obtained by direct assay (kocat) and from fits of data in Fig. 1 to equation (1).
In summary we have investigated the kinetics and thermodynamic activation parameters of a number of different hybrid tetramer systems comprising WTRePC subunits and mutant RePC subunits. Our studies show the importance of biotin and the BCCP domain in the organization of the structure and function of RePC tetramers, and also indicate the proton relay formation in the BC domain of binding of MgATP also plays a role in tetramer organization. The data of WTRePC:D1018A.K1119A hybrids suggest that where there is only one or two WTRePC subunits on a face of a tetramer, with catalytically inactive subunits on the other face, obligatory oscillation between asymmetrical and symmetrical conformers of the tetramer does not occur. Finally, we showed that, hybrid tetramer formation can occur between pyruvate carboxylase subunits from evolutionary distant species that retain highly conserved residues involved in subunit-subunit interactions.
| Relevant SDGs | |
|---|---|
 |
|
| BC investigator | |
 Prof.Sarawut Jitrapakdee Prof.Sarawut Jitrapakdee |
 Khanti Rattanapornsompong (Ph.D. Student) Khanti Rattanapornsompong (Ph.D. Student) |