Protonation status and control mechanism of flavin–oxygen intermediates in the reaction of bacterial luciferase
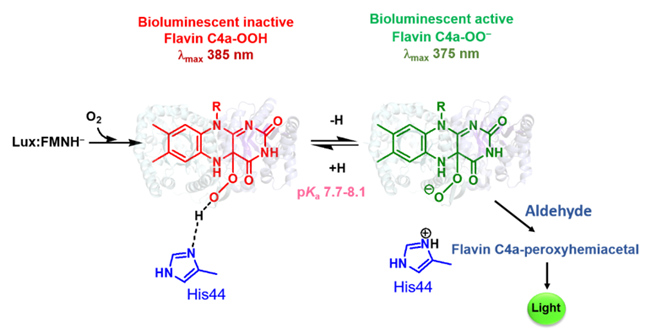
Bacterial luciferase catalyzes bioluminescent reaction that has long been used for sensitive detection tools in biomedical, food, and environmental applications. In this research, we elucidated crucial factor to control the protonation of intermediate that determines the light output of bacterial luciferase reaction. Our finding showed that the bioluminescent reaction proceeds by generating a reactive intermediate of flavin C4a-oxygen adduct and the intermediate is first generated in a protonated form of flavin C4a-hydroperoxide, which is unable to react with an aldehyde. The active site His44 functions as an essential proton abstractor to convert the flavin C4a-hydroperoxide to flavin C4a-peroxide that is capable to proceed the bioluminescent reaction. This is the first identification of mechanistic control of the protonation state of flavin intermediates in a nucleophilic flavin‐dependent monooxygenase. Understanding how the protonation of flavin C4a‐OOH can be controlled is important for fine‐tuning the activity of nucleophilic or electrophilic monooxygenases.
Reference:
Tinikul R, Lawan N, Akeratchatapan N, Pimviriyakul P, Chinantuya W, Suadee C, Sucharitakul J, Chenprakhon P, Ballou DP, Entsch B, Chaiyen P. Protonation status and control mechanism of flavin–oxygen intermediates in the reaction of bacterial luciferase. FEBS J. https://doi.org/10.1111/febs.15653
| Relevant SDGs | |
|---|---|
 |
|
| BC investigator | |
 Asst. Prof. Ruchanok Tinikul Asst. Prof. Ruchanok Tinikul |
